-

Bird flu is spreading to more farm animals. Are milk and eggs safe?
A bird flu outbreak in U.S. dairy cows has spread to more than two dozen herds in eight states. That comes weeks after the nation’s largest egg producer found the virus in its chickens. Health officials continue to stress that the risk to the public is low and that the U.S. food supply remains safe and stable.
-

Many cancer drugs remain unproven 5 years after accelerated approval, a study finds
Researchers have found that most cancer drugs granted accelerated approval by the U.S. Food and Drug Administration do not deliver on their early promise.
-

With the first ever over-the-counter birth control pill, DE lawmakers are working to protect women who use it
The first ever FDA-approved over-the-counter birth control pill is about to go on sale at pharmacies across the country. Delaware lawmakers are working to make sure insurance companies will cover the costs. NBC10’s Tim Furlong has more.
-

FDA warns about lead contamination in more cinnamon products in the US
The Food and Drug Administration issued a safety warning Wednesday, saying it identified additional cinnamon products in the United States that are contaminated with lead.
-

Watch out for fake Ozempic shots that are being sold through legitimate drug supply sources
The U.S. Food and Drug Administration says it has seized “thousands of units” of counterfeit Ozempic, the diabetes drug widely used for weight loss.
-

Lead contamination in applesauce pouches may have been intentional, FDA says
The lead contamination in recalled cinnamon applesauce pouches that potentially poisoned at least 65 children may have been intentional, the Food and Drug Administration said on Friday.
-
After recalls and infections, experts say safer eyedrops will require new FDA powers
Repeated recalls of eyedrops are drawing new attention to the limited powers U.S. regulators have to oversee medical products made overseas. Unlike prescription drugs, eyedrops and other over-the-counter products don’t get preliminary review by the Food and Drug Administration. In the case of two eyedrop recalls earlier this year, FDA inspectors had never previously visited the plants in India. In...
-
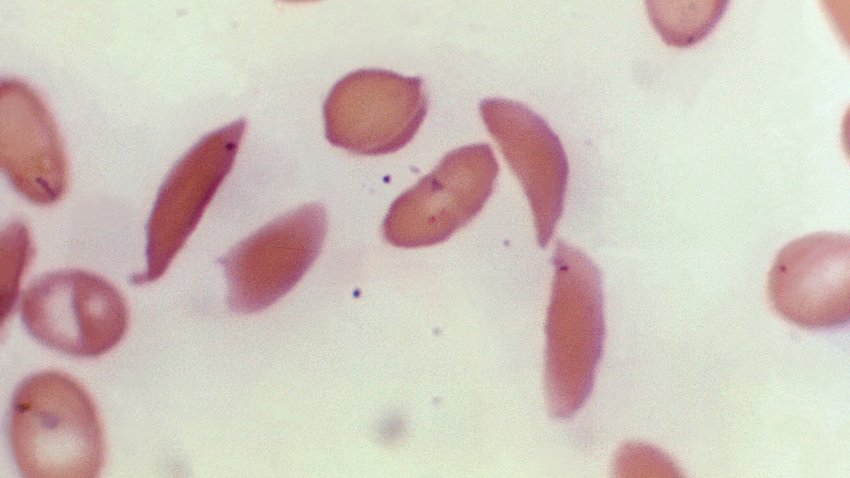
FDA approves two sickle cell disease therapies, including world's first gene-editing treatment
U.S. regulators have approved two gene therapies for sickle cell disease.
-
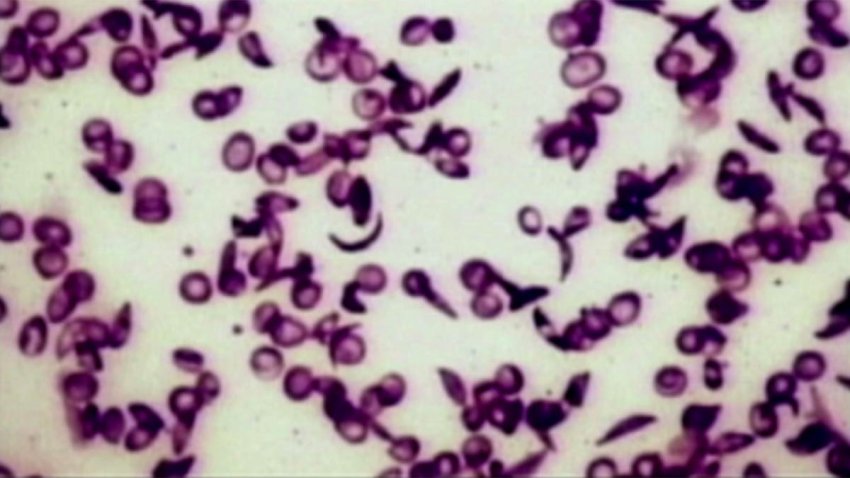
FDA approves groundbreaking treatments for sickle cell disease
One of the treatments uses CRISPR gene-editing technology.
-

FDA screening US cinnamon imports after more kids sickened by lead-tainted applesauce
The U.S. Food and Drug Administration is screening imports of cinnamon from multiple countries for toxic lead after reports of illness in children who ate pouches of applesauce puree.
-
Barefoot workers and cracked floors were found at a factory that made recalled eyedrops, FDA says
U.S. health inspectors found a host of sanitation and manufacturing problems at an Indian plant that recently recalled eyedrops sold in the U.S.
-

FDA proposes ban on food additive found in fruity sports drinks and sodas
The Food and Drug Administration on Thursday proposed banning the use of brominated vegetable oil, a food ingredient once widely used in popular drinks like Gatorade and Mountain Dew that has been slowly phased out due to its link to potential health risks, including damage to the liver, heart and brain.
-

FDA warns consumers to stop using 26 eye drop products sold at CVS, Target and Rite Aid
The FDA is warning consumers to immediately stop using 26 over-the-counter eye drop products due to the “potential risk of eye infections that could result in partial vision loss or blindness.”
-
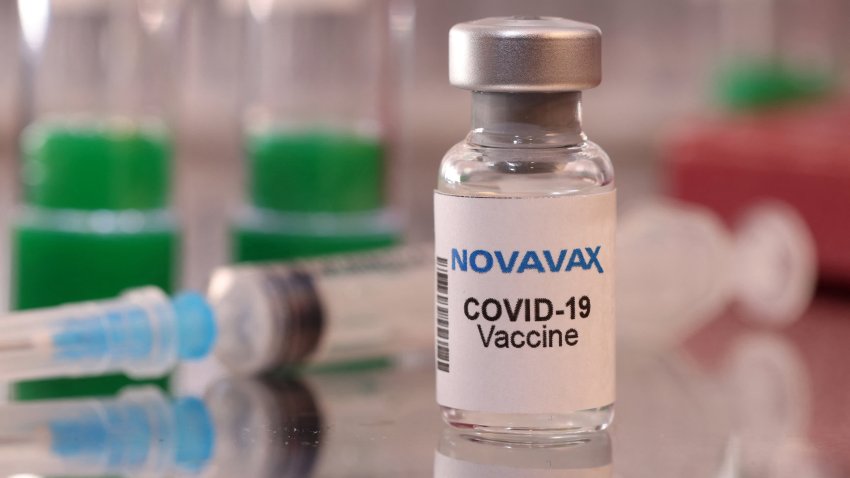
Novavax updated Covid vaccine wins FDA, CDC backing, paving way to reach Americans within days
Health officials see Novavax’s protein-based vaccine as a valuable alternative for people who don’t want to take messenger RNA shots from Pfizer and Moderna.
-
After US approval, Japan approves Leqembi, its first Alzheimer's drug
Japan’s health ministry has approved Leqembi, a drug for Alzheimer’s decease that was jointly developed by Japanese and U.S. pharmaceutical companies.
-

FDA skeptical of experimental ALS treatment pushed by patient advocates
NurOwn, a stem cell therapy, is at the center of a yearslong lobbying campaign by patients seeking access to experimental medicines. FDA regulators say the treatment hasn’t been shown to work.
-

FDA rejects first needle-free alternative to EpiPens, calling for additional research
The move came as a surprise: In May, an FDA advisory committee voted to recommend approval of the drug for children and adults.
-

How to read a Nutrition Facts label
These labels are found on most pre-packaged food items in the United States. They’re loaded with important information that can be difficult to decipher. Here’s how to read one of these labels.
-

FDA panel says this popular over-the-counter decongestant doesn't actually work
If the FDA follows through on the panel’s recommendations, Johnson & Johnson, Bayer and other drugmakers could be required to pull their oral medications containing phenylephrine from store shelves.
-
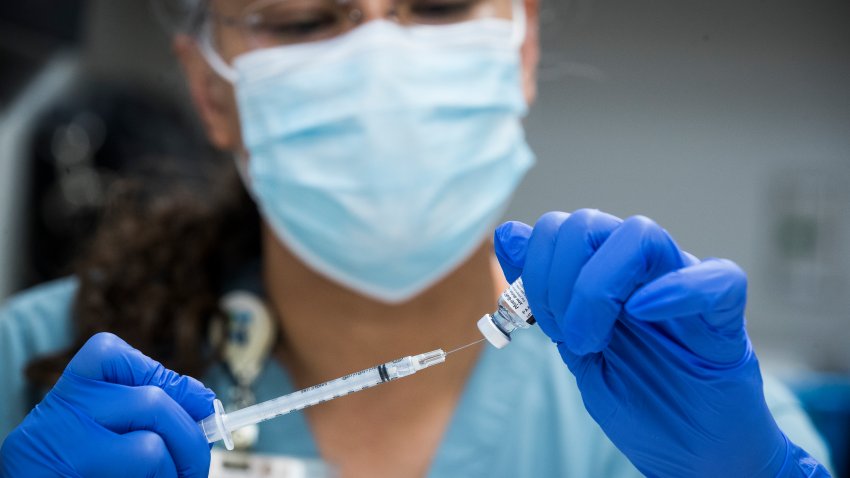
CDC recommends updated Covid shot for everyone ages 6 months and up
A panel of CDC advisers voted Tuesday to recommend updated Pfizer and Moderna coronavirus booster shots for everyone ages 6 months and up.

